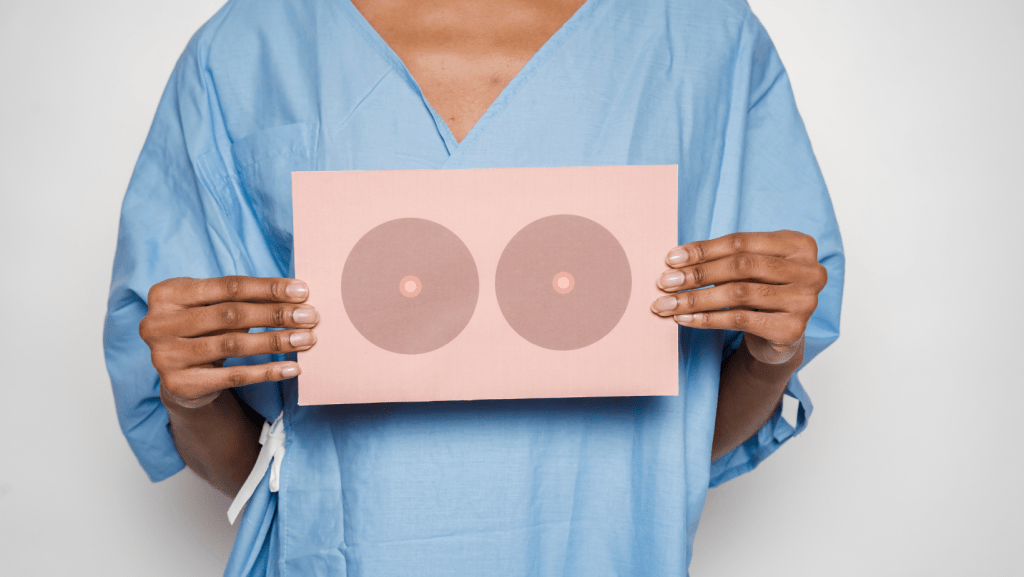
The Food and Drug Administration placed a “black box” warning on breast implants to advise patients of health risks and side effects, according to the New York Times. Federal regulators ordered manufacturers to only sell implants to providers who agree to communicate all the risks to patients prior to surgery. The warning, along with a new checklist for patients and providers to review before surgery, states that breast implants have been linked to several serious health conditions, including a cancer of the immune system and several autoimmune diseases.
E-cigs linked to weaker bones
Use of e-cigarettes -- also known as "vaping" -- is linked to increased risk of hip, spine and wrist fractures, according to US News & World Report. Researchers at the University of Pittsburgh examined data from more than 5,500 health survey participants and found...